

- #CHARGE OF CARBON IN CO3 HOW TO#
- #CHARGE OF CARBON IN CO3 FULL#
- #CHARGE OF CARBON IN CO3 DOWNLOAD#
Furthermore, there is not a full negative charge on any of the oxygens but about.
#CHARGE OF CARBON IN CO3 DOWNLOAD#
Now you score 5+15 POINTS by uploading your Pic and Downloading the Askiitians Toolbar respectively : Click here to download the toolbar. In fact, carbonate really has about 1-1/3 bonds between each carbon and oxygen.

Carbon single bonded to one oxygen and double bonded to another (carbon +1, oxygendouble 0, oxygensingle 1, total. Carbon single bonded to both oxygen atoms (carbon +2, oxygens 1 each, total formal charge 0). There are three different ways to draw the Lewis structure.
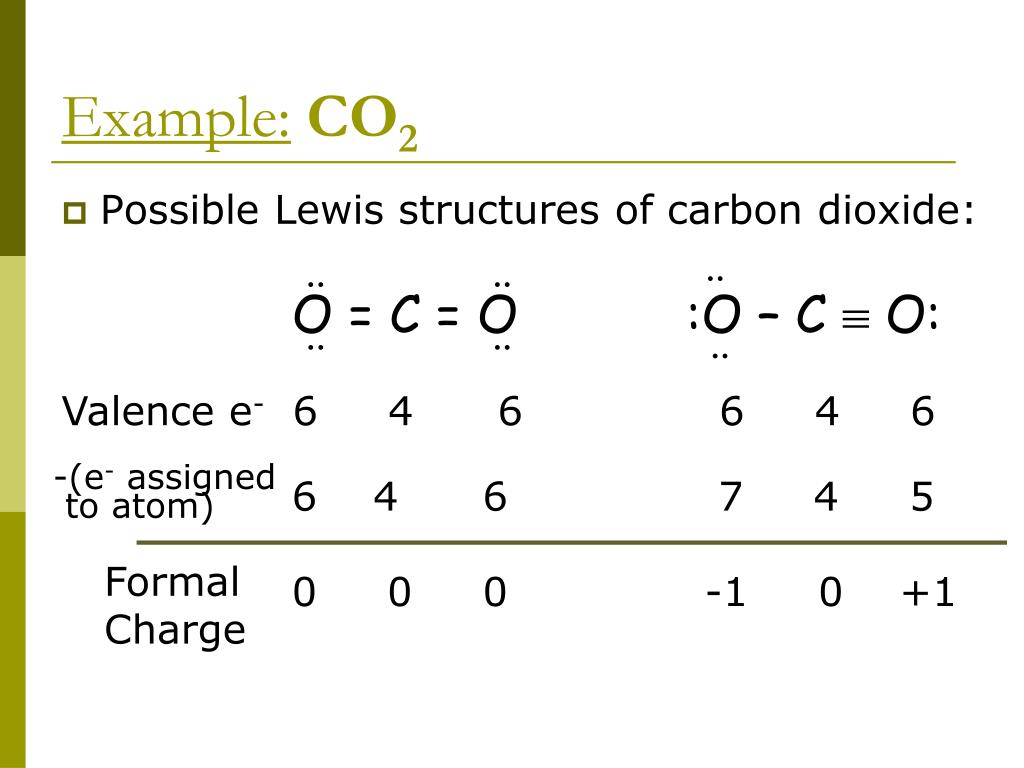
So help discuss any query on askiitians forum and become an Elite Expert League askiitian. CO 2 is a neutral molecule with 16 total valence electrons. Here the charge is 2 Oxygen typically has an oxidation number of 2, and it does here. The sum of the formal oxidation numbers, in a complex or compound ion, must sum of the charge of the ion.
#CHARGE OF CARBON IN CO3 HOW TO#
Win exciting gifts by answering the questions on Discussion Forum. How to Calculate the Formal Charges for CO3 2- (Carbonate ion) Wayne Breslyn 681K subscribers 50K views 4 years ago In order to calculate the formal charges for CO3 2- we'll use the. Therefore, the oxidation state of C,is IV. We are all IITians and here to help you in your IIT JEE preparation. Please feel free to post as many doubts on our discussion forum as you can.
single bonded oxygen in NO 2 - FC = 6 - 6 - (2÷2) = -1 Carbon trioxide can be produced, for example, in the drift zone of a negative corona discharge by reactions between carbon dioxide (CO 2) and the atomic oxygen (O) created from molecular oxygen by free electrons in the plasma. double bonded oxygen in NO 2 -: FC = 6 - 4 - (4÷2) = 0. Nitrogen in NO 2 -: FC = 5 - 2 - (6÷2) = 0 The carbon, in the carbonate ion, has 4 x 1 4 electrons assigned to it (one from each of its four bonds), therefore it has a formal charge of zero (neutral). When determining the correct Lewis structure (or predominant resonance structure) for a molecule, the structure is chosen such that the formal charge (without sign) on each of the atoms is minimized. Calcium Carbonate CaCO3 or CCaO2 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Where V is the number of valence electrons of the atom in isolation (atom in ground state) N is the number of non-bonding valence electrons on this atom in the molecule and B is the total number of electrons shared in covalent bonds with other atoms in the molecule. Chemical equilibria within the ocean then fix the amount of bicarbonate : CO2 + CO3 +. The formal charge of any atom in a molecule can be calculated by the following equation: carbon the CO2 is fixed by the economics of the excess cations. Concepts of Physics by HC Verma for JEEĪ formal charge (FC) is the charge assigned to an atom in a molecule, assuming that electrons in a chemical bond are shared equally between atoms, regardless of relative electronegativity. IIT JEE Coaching For Foundation Classes.







 0 kommentar(er)
0 kommentar(er)
